BACKGROUND: More than 60% of sickle cell anemia (SCA) adults develop chronic kidney disease (CKD). Identification of early stages of CKD in SCA patients at high risk of complications could lead to personalized treatment and better prognosis. Recently we identified several urinary biomarkers that can differentiate CKD stage 1 in SCA patients. These biomarkers reflect pathophysiology of SCA, including iron homeostasis (ceruloplasmin, transferrin, hemoglobin, and ferritin); inflammation (orosomucoid); and glomerular hyperfiltration (hepatocyte growth factor like).
HYPOTHESIS: We hypothesized that combination of novel urinary biomarkers might improve the accuracy of early detection of CKD.
METHODS: We evaluated spot urine samples of 54 patients with SCA in a steady state from the University of Illinois at Chicago. Patients were classified by the stage of chronic kidney disease (CKD) based on the National Kidney Foundation, Kidney Disease Outcomes Quality Initiatives guidelines. Three groups of CKD stages were compared: Stage 0 (without CKD, n=23), stage 1 (early stage of CKD, n=19), and stages 2 to 5 (moderate to severe CKD, n=12). Urine levels of ceruloplasmin (CP), transferrin (TrF), hemoglobin (Hgb), ferritin (FrT), orosomucoid (ORM), and hepatocyte growth factor like (HGFL) were measured by ELISA and normalized to urinary creatinine (CRE) concentrations. Differences in sensitivity and specificity of biomarker combinations were compared using the McNemar's Test against the simple model, which uses urine Hgb only, and the complete model, with all the biomarkers together. A test for the equality of the Area Under the Curve (AUC) compared to the simple and complete models was done using the algorithm suggested by DeLong, DeLong, and Clarke-Pearson (1988).
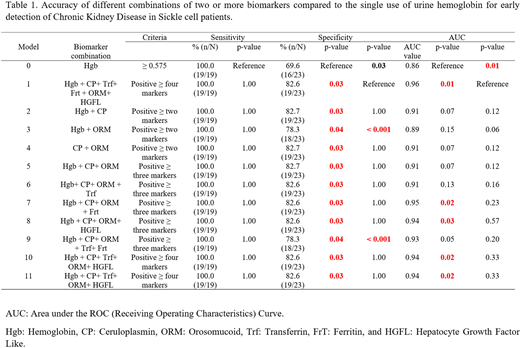
RESULTS: We tested the ability of each biomarker to distinguish SCA patients without CKD and with CKD stage 1. Receiver operating characteristic (ROC) curves were constructed to determine appropriate cutoffs for each biomarker. Cutoffs that provided the highest Youden Indexes were used. Three biomarkers (Hgb, CP, and ORM) had a sensitivity of 100%; however, specificity was lower than 80% (range: 65.2%-72.7%). Other biomarkers (Frt, Trf, and HGFL) had sensitivities lower than 80%, suggesting that individually these biomarkers are not accurate for early detection of CKD.
We compared different combinations to the single biomarker model (Hgb only or model 0) and a complete combination of six biomarkers (model 1) (Table 1). The complete combination significantly improved specificity (from 69.6 to 82.6%) and increased AUC (from 0.86 to 0.96) compared to the single biomarker model. All combinations significantly increased the specificity (from 69.6% to 78.3-82.7%) compared to the single biomarker model. Combinations of four to five biomarkers (models 1-6) significantly increased specificity and AUC values than model 0. Combination of Hgb + CP+ ORM + Frt produced similar specificity and AUC values compared to complete model.
CONCLUSION: These results demonstrate that the use of multiple biomarkers can improve the accuracy in the detection of kidney pathology in SCD patients, which has essential value for clinicians and researchers of clinical trials to target high-risk individuals for early treatment and preventive care.
LIMITATION: Small cohort of patients from one center.
Saraf:Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Advisory Boards, Speakers Bureau; Novartis, Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pfizer, Global Blood Therapeutics, Novartis: Research Funding. Gordeuk:Imara: Research Funding; Novartis: Consultancy; Ironwood: Research Funding; CSL Behring: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal